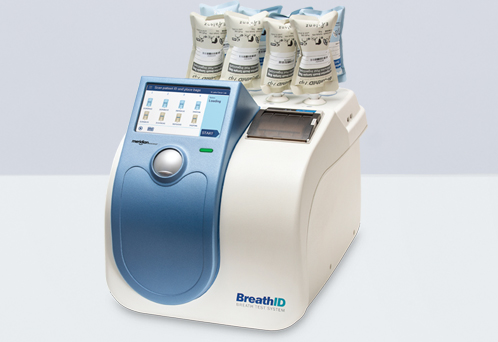
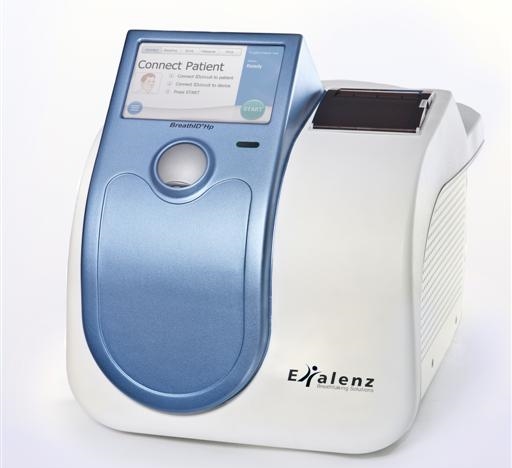
The breathid hp breath test system is used to perform a diagnostic test for the presence of helicobacter pylori a bacterium that can cause peptic ulcers and is associated with gastric cancer.
Breathid hp breath test system.
Pylori a type of bacteria that may infect the stomach and is a main cause of ulcers in both the stomach and duodenum the first.
One package insert one tablet of 13c enriched urea 75 mg.
If desired use the provided sample transport bag for transport of the breath samples.
Other applications are limited in the us by federal law to investigational use only.
In the us the breathid hp system and the breathid hp lab system are intended for use in the qualitative detection of h.
The urea breath test is used to detect helicobacter pylori h.
In the us the breathid hp system and the breathid hp lab system are intended for use in the qualitative detection of h.
They have fda 510 k clearance and an approved nda for the 13 c urea tablet and citrica powder.
Listing a study does not mean it has been evaluated by the u s.
They have fda 510 k clearance and an approved nda for the 13 c urea tablet and citrica powder.
Blood chemistry leadcare proficiency and controls human conditions.
H pylori is a very common bacterium reported to be present in up to two thirds of the world population.
Urea breath test ubt with breath hp system breathid hp lab system pediatrics the safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
Each idkit hp two for the exalenz breath test with the breathid hp lab system contains.
Materials a single breathid idkit hp two is provided to perform the breath test.
Other applications are limited in the us by federal law to investigational use only.
The exalenz breathid hp lab system is indicated for use as an aid in the initial diagnosis and post treatment monitoring of h pylori infection in adult patients and pediatric patients ages 3 17 years old.